Inclined Bed Therapy: Sleeping Inclined To Restore and Support Your Health For Free. Fascinating Science, Discovery, History and Medical Research In Circulation And Posture, by Andrew K Fletcher. Read the Success Stories. Check the Forum.
Inclined Bed Therapy: Sleeping Inclined To Restore and Support Your Health For Free. Fascinating Science, Discovery, History and Medical Research In Circulation And Posture, by Andrew K Fletcher. Read the Success Stories. Check the Forum.
How do Trees Really lift Water to their Leaves?
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

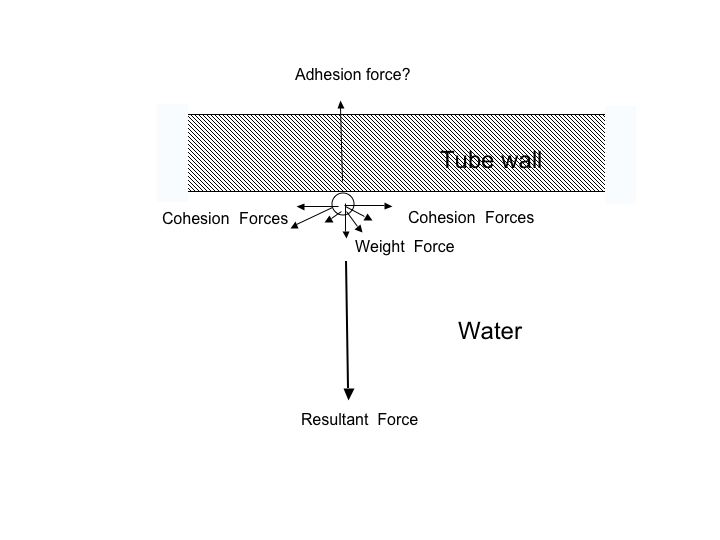
You say water would move away from the inside of the tube. As water is already compressed by gravity, where is it going to move to? It can't move down the tube because it is linked to other molecules balancing the downward pull equally on the opposite side of the U tube. It can’t flow out because this would require cavitations to form, again to break the cohesive bond. It can’t flow up because of gravity, It can’t collapse the tube because the wall is strong enough to resist the decompression. If molecules move away from the wall, more molecules must replace it because each molecule acts upon its neighbours. Cavitations will inevitably combine to form larger vapour bubbles and this will cause the water columns to fall back to the level at which atmospheric pressure can sustain them @ 10 metre mark, so although the molecules cannot determine which experiment they are in, we must take into account at all times that each molecule is linked to another inside the tube, so deciding the fate or purpose of a single molecule or groups of molecules that are part of a huge volume of molecules in the same experiment is a little difficult to consider to say the least.
I have now read the lengthy paper you linked to and found it to be a report on the progress of science relating to cohesion, adhesion, tension, surface tension and cavitations in water and other various liquids, dealing with ancient science and more modern science approaches to the problems of cavitations in water. Most of which was familiar to myself, and some of which I have included in the two threads, including the spinning tubes, stretched water, etc. Nevertheless, I have not seen this paper before and it does include at least two experiments that I have not heard of before. So thank you for posting the link and for taking the time to look at the experiments.
The terminology I use may not always be in accordance with writing a convincing academic argument and I agree with you on this at least, but if you compare what I have stated to what you have provided in the PDF file we are not far from the mark at all.
Throughout the paper you provided, there is no mention of timescales for cavitations to develop in degassed water, although nucleation is mentioned in relation to impurities in water, I suspect the main seed points are indeed between the water/tube interface. I cannot see another experiment that could show timescales for cavitations other than inside a living plant or tree. The U tube gives us prolonged stability of water under negative pressure and tension allowing us to see cavitations developing through the opaque tubing. There must be clearer tubing produced that could allow us to look more clearly at the forming cavitations.
Deionised water previously boiled will enable the Brixham experiment to easily exceed the 24 metre mark and give us prolonged stability.
I thought the inclusion of particles entering the experiment to cause the nucleation was interesting also. And had not considered this.
The following history event shows how a tiny bubble of gas causes the Huygens experiment, (similar to yours), fails from the paper you provided the link to.
4.1. Pull
A straightforward way to stretch a liquid is to pull directly on it. The pull can be generated by the own weight of the liquid. This is how Huygens made the first experimental observation of negative pressure in 1662, and published his work in 1672 [49]. A tube open at one end is filled with water purged of air, and inverted over a water bath. If the air above the bath is evacuated, water remains suspended in the inverted tube. The pressure at the top of the water column of height h is Psat − ρgh, where ρ is water density, and g the acceleration of gravity. As soon as a bubble of air is injected in the tube, it rises and the water column falls. This experiment was presented to the Royal Society of England, and repeated on water and mercury by several physicists, including the famous Hooke and Boyle,
who reached −0.2 MPa in mercury. The phenomenon was later re-discovered by Donny [23] and Reynolds [47,48]. Details are given by Kell [50]. As Reynolds used a 2.3 m-long tube wetted with water before being filled with mercury, he obtained the most negative pressure for water with this method: −0.3 MPa [48]. Hayward, who thought that the method was invented by Donny [23], re-used it to study different liquids [51]. Another way to pull a liquid is to mechanically increase its volume with a bellow for instance. One can also put the liquid under pressure before warming it up, and eventually releasing the pressure. These techniques have been widely used to make bubble chambers where high energy particles are detected because they trigger cavitation in the metastable liquid (see Ref. [53] for a review); however, volatile liquids with a low surface tension were preferred to water. But the bellow method was used by Hayward to design a water pump with a suction lift of 17 m, corresponding
to a pressure of −0.17 MPa [52]; much higher liquid columns exist in tall trees (see Section 7.1).
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

Saying that you are thinking laterally doesn't prove that you are right.
You have brought up many issues which are very interesting and have changed my views about the behaviour of water under negative pressure.
BUT the model which is in your head is still flawed. It cannot hope to describe what is going on if you say, for instance, that you have negative pressure up there but gravity is still Pushing the water against the tube. How is that consistent?
A truly valid model has to deal with all the little nitpicking details which you choose to ignore. You cannot be so selective about what you do and don't accept about convention without total rigour. Your explanations for what happens in your experiment just would not extend to other situations - even to a single tube. Your brief experiment was not detailed enough. You won't even answer my reasonable queries about it. You are too confident of your model to submit it to scrutiny. Do you not see how important it is to make clear what was in the void at the top of the tube? If it is air then there must be a leak. If there is water vapour or a vacuum then the void would vanish when pressure increases again. What happened? Can you remember? It is NOT irrelevant if your interest is truly scientific. There is nothing glamorous about pressing on and ignoring these queries and objections.
I despair of ever getting answers to these and the other questions which I and others have asked. If you want to be ground-breaking you have to be totally thorough.
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

I'm sorry; were the following extracts not direct enough?
Quote from my post of 29 Oct:
Your original post did not discuss how the space at the top had formed. Are you suggesting that the gap at the top of the tube was a vacuum? In which case , when you removed your 'extra force', the water would have gone back up to the top (as Galileo et al have found). If the gap remained, then it was full of air. This air can only have got there as bubbles from the bottom, out of solution or via a leak in your 'sealed end. It must have come from somewhere.
Quote from my post of 29 Oct (later):
How about my question regarding the space that you saw above the column of water? Did it stay there? Was it air? How did it get there? There is an interesting practical question here.
My comments referred to the single tube - to avoid confusion. It was clearly in that context. Your last answer seems to refer to the high level U tube experiment. You see, I think that you really don't want the single tube to work because it would go against your theory. However, if you can clear up this problem then I would take that back, of course.
Did you also not read my direct statement that you were wrong when you wrote the tension is the same throughout the column? That needs an explanation from you, I think.
I also made a direct comment, earlier, using chewing gum as a metaphor for water at the top of a tube. No comment about that, either; was it irrelevant?
I just saw this statement from you whilst I was revisiting past posts:
"Stop thinking of water as water, start thinking of it as a solid."
Were you joking? Can that be taken seriously? At what point do we have to treat a bowl of water as a bowl of solid? You could freeze it - but I don't think that's what you meant. When does a water molecule, at room temperature, know how to treat its neighbours differently? When is it part of a solid and when is it part of a liquid?
Can I recap on your recent single tube experiment? As I understand it you used a single tube, less than 10m long and whirled it around to simulate a longer tube. You noticed cavitation at the top whilst it was revolving and this cavitation disappeared when you stopped. IS this correct?
What was the actual length and at what speed did you rotate the tube? How did you see the effects?
I'm not sure but I got the impression that you repeated this with a U tube and the water ran out. This is what you'd expect because of asymmetry.
Last comment, for now: Could you please define what Density Flow means? I can't find it anywhere apart from in your writings.
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

Sorry but I just spotted this in your last post.
You say water would move away from the inside of the tube. As water is already compressed by gravity, where is it going to move to? It can't move down the tube because it is linked to other molecules balancing the downward pull equally on the opposite side of the U tube. It can’t flow out because this would require cavitations to form, again to break the cohesive bond.
I was 'indoctrinated' into mechanics when at School, along with Newton's Laws. Forces add vectorially (would you not agree?).
At the top of the tube (when it is >10m, to keep the diagram simple), we have cohesive forces, acting towards all the nearby water molecules and the weight of the molecule, acting downwards. Nothing is being 'compressed by gravity', the molecule is just being pulled downwards by its own weight. The other molecules are all pulling away from it. These all produce a resultant force which acts away from the wall. Unless this force is cancelled by an equal and opposite adhesion force, the molecule will move away from the wall. It can only stay where it is when the forces are balanced. Can you possibly disagree with that?
When this molecule moves away, it, of course, would let all the other molecules move - they are under tension- and they can flow out of the bottom of the tube as long as there is an unbalance in the forces.
Unless you have adhesion - not just a bit, but of equal value to balance the other forces involved - the bead will part company with the wall. If you don't agree with that then you need to go away and learn the basics of how forces work.
Needless to say, this applies to any shape of container, be it U tube, or an upside down Poseidon in the well known film.
You don't have to pick me up on this example because, in that case, the adhesive forces are not enough AND cavitation will occur. But that isn't the point of the argument. The point is that, if the column stays up, it must involve both 'strong' adhesion and a delay in cavitation.
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

A video on Youtube shows the flow through a clear glass vase. Watching it you could easily miss the point that for a downward flow of denser solutes, there must be a return flow to the surface, water molecules will always move to where molecules have moved from, just the same as they would when molecules move away from the inside of the pipe.
Your post asking about the void related to the single capped tube. I said that water flowed out of the tube when the experiment was performed, so could not have returned to fill up the void.
Damn, just realised a mistake with that capped experiment. Maybe if we have a single capped end of tube and the open end in a vessel rather than being exposed to the air will assist the water to resist the centrifugal force longer. This would require affixing the bottle in some way to the end of the tube to prevent it from flying off.
There was no point swinging round a U tube in the same way because water would not remain in it. Again however if the both ends of the tubes were in a container and it was swung round it may prove interesting.
The chewing gum analogy necking, I did mention that as one molecule moves away from the wall of the tube another will replace it providing of course that the cavitation is not sufficient to cause the bead of water to fail.
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

OK on the term 'density flow'. In a hot water (convection) system, the energy comes in the form of thermal energy. To keep it going you need to keep the energy flow by maintaining a temperature difference.
The heat source alters the density of the water causing it to rise.
That's schoolboy howler number one. Water will not rise unless it is pushed. There are no strings pulling it up. What pushes it? It is the more dense cold water which displaces it.
Andrew, if you are not thorough with well known matters like that then how can you hope to make any worthwhile advances in Science?
Your post asking about the void related to the single capped tube. I said that water flowed out of the tube when the experiment was performed, so could not have returned to fill up the void.
So what was in the space over the water? If there was a permanent 'void' at the top then it must have been AIR! That means either a leak into the top or bubbles floating up from the bottom. I wish you could explain exactly what happened. It certainly casts doubt on the experiment.
water molecules will always move to where molecules have moved from,
Here's another cause and effect problem. The more dense solution displaces the less dense. Why? Because the more dense is pulled down harder than the less dense and pushes it out of the way. When you add the solution, you increase the overall pressure at the bottom of the container.
How does that apply to molecules as they move from the surface when water evaporates? You imply that you would get 'strings of water' leaping up into the air.
I did mention that as one molecule moves away from the wall of the tube another will replace it
And where does this molecule come from? Does there have to be a flow? Do you inject them into the top? Get your model sorted out; it really is dodgy. Or are you only considering the situation when you have enough gum flow to allow the molecules to come from the other side? You are implying that there is a minimum speed at which this would work. Any slower and the gum will 'neck'. My diagram applies to the gum just the same as the water. Are you arguing with the basics of force vectors?
« Last Edit: 03/11/2008 19:03:32 by sophiecentaur »
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

Your statement that water will not rise unless it is pushed may need a little edit, else you will have the entire cohesion tension fringe of science after you. They state as one molecule leaves a leaf (scuse the pun) another is pulled behind it to replace it and this believe it or not is largely accepted science and all science has to offer with regards to the ascent of sap in tall trees, perhaps you should take a leaf out of their books?
And yes there are strings pulling on the fluid, strings of particles linked together just like RD suggested in his chain analogy. Using food colouring we can see how the flow occurs, we do not see a uniform full bore flow but a considerable amount of turbulence as some of the coloured salt solution is flowing down the return flow side of the inverted U tube which rolls in a circular motion as salt free water is drawn past it. Fascinating to watch by the way and well worth studying. It shows how molecules pull on each other too. What do you suggest might be pushing water out of the top of a giant sequoia towering well over a hundred metres? Root pressure? Magic?
The central heating system shown was merely to illustrate that a downward flow will result in a return flow. But more to the point to coin one of your phrases; ‘How do molecules of water know they are in a U tube, a central heating system, a single upright capped tube, a tree or indeed a human ’? Why should we expect that an upward flow or downward flow inside a tree will not produce a return flow? Schoolboy stuff this, but before the education system gets in their way!
And let us not forget your meniscus example where adhesion is pulling the water up at the edges, or have you forgotten this argument already?
I have not said there is a minimum flow at which this will work. I have said that we have an excellent opportunity to study cavitation in this model due to it’s stability and this is the first time the speed at which cavitation takes place in water suspended in a meta-stable state. There is another thing I would like to add. It would appear that cohesion is taking place in the upward flowing leg of the suspended tube. I may certainly have missed some cavitations forming in the 24 meter’s of water filled tubing on the down flow side, but suspect that the saline flows, which represents the phloem in trees is repairing the voids? Or is the positive pressure evident by the outflow from the bottle at ground level sufficient to prevent them from occurring in the down flow side? Even the bench top model produces cavitations over time.
The rotating tube failed because the open end allowed all the water to come out of the tube emptying it completely so unable verify if there was void in it or not.
To conduct a 24 meter single tube experiment would be a pain in the back side. Have you ever tried filling a six mil bore tube with water and making sure there are no bubbles in it, that is capped at one end? I have, and will not be attempting to fill a 24 meter one.
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.
- Andrew
-
 Topic Author
Topic Author
- Offline
- Moderator
-

So there is an attraction to the wall of the tube and molecules should align to the tube to form adhesion by using the opposite polarity to the nylon tube molecules-whether this is relevant at the moment I’m not sure. Nevertheless water can flow out either side of the tube, so adhesion does not prevent the outflow in the U tube. So cohesion must be the main stabilising force. What I hope I have said here is that although the water is stuck to the inside of the tube, water can move freely it does not arrest the water column so cannot be responsible for holding the open ended water filled tubes that we have exposed to the atmosphere by removing them from the water. So why does the water not flow out? What explanation other than the elasticity of water which is related directly to the cohesion of water will account for the water rapidly rising up the exposed tubes forming a level a considerable distance from the ends of the tubes?
Gravity, Learn to live with it, because you can't live without it!
Please Log in or Create an account to join the conversation.